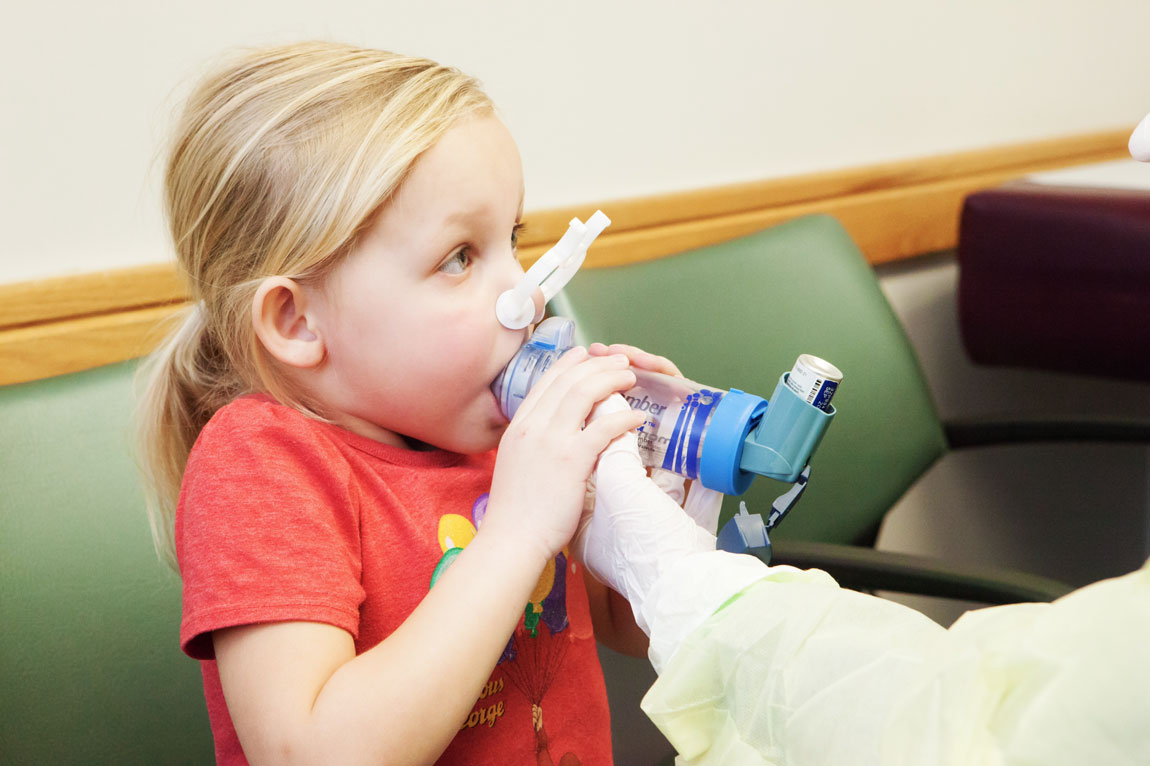
Research
Major research efforts within the Pulmonary and Sleep Medicine Division involve various clinical studies, many of them dealing with patients with cystic fibrosis. Many of these studies deal with new and novel treatments for children with this life-shortening disease.
Therapeutics Development Network
The pediatric Cystic Fibrosis Center at Intermountain’s Primary Children’s Hospital is a member of the Therapeutics Development Network (TDN). The Therapeutics Development Network (TDN) is a nationwide network of nearly 80 cystic fibrosis clinical research centers. These centers specialize in conducting clinical trials to evaluate the safety and effectiveness of new CF therapies.
The TDN is primarily funded by the Cystic Fibrosis Foundation Therapeutics and occasionally by private industry sponsors and the National Institutes of Health. The TDN is involved in various trials including gene therapy, protein-assist therapy, and anti-infective agents.
Research Projects
The pediatric pulmonology research staff is currently participating in several projects:
- The EPIC Observational Study is looking at early antipseudomonal treatment in children with cystic fibrosis. We are in year eight of the 10- year study.
- The VX08-770-105 clinic trial is an open-label study to evaluate the long-term safety and efficacy of VX-770 in subjects with cystic fibrosis. VX-770 is a CFTR potentiator that aims to increase the ability to transport ions across the cell membrane.
- The iCARE study is testing two potentially effective adherence interventions for adolescents and young adults in hopes of improving compliance of medication use and prescribed therapies. The Twin/Sibling study looks at genetic, environmental, and clinical status of twins and siblings with CF to determine possible genetic modifiers of the disease.
- The Mpex207 & Mpex209 studies are testing the safety and efficacy of inhaled Levofloxacin as compared to Tobramycin Inhalation Solution in the 209 trial and Placebo in the 207 trial.
For more information about clinic trials, please contact Jane Vroom, CCRC at 801-587-7458 or Heather Oldroyd at 801-587-7457.