The ability to accurately and reliably model ultrasound beam propagation is a crucial component of MRgFUS research. Modeling patient-specific treatments allows us to examine potential phase aberration effects and ways to correct for those effects, all before actually using the technology in an experiment or with a patient. Accurate acoustic modeling can result in safer and more effective treatments. It is important to be able to accurately model how a focused ultrasound beam propagates through the inhomogeneous tissue structures of the human body. Our lab group has developed a technique known as the hybrid angular spectrum (HAS) technique, which is an extension of the more traditionally used angular spectrum method. HAS can successfully model linear wave propagation and can account for the inhomogeneous structure of human tissues. Our current research is quantitatively validating our acoustic simulation method in ex vivo and in vivo environments and will be integrated into Thermoguide, a MRgFUS clinical treatment environment developed by Image Guided Therapy, Inc.
.png)
Related Publications
Vyas, U., & Christensen, D. (2012). Ultrasound beam simulations in inhomogeneous tissue geometries using the hybrid angular spectrum method. IEEE Trans Ultrason Ferroelectr Freq Control, 59. doi:10.1109/tuffc.2012.230
Johnson, S. L., Dillon, C., Odeen, H., Parker, D., Christensen, D., & Payne, A. (2016). Development and validation of a MRgHIFU non-invasive tissue acoustic property estimation technique. Int J Hyperthermia, 32(7), 723-734. doi:10.1080/02656736.2016.121618
Vyas, U., & Christensen, D. A. (2011). Extension of the angular spectrum method to calculate pressure from a spherically curved acoustic source. J Acoust Soc Am, 130(5), 2687-2693. doi:10.1121/1.362171
Phase Aberration Correction
Focused ultrasound (FUS) is a completely non-invasive treatment method that uses high-intensity, focused sound waves to treat various disease pathologies. When FUS is applied to biological tissues, the ultrasound waves are absorbed by them, converting the mechanical energy of the sound waves into heat. In order to ensure the safety and efficacy of these ablation treatments, clinicians need to measure the rise in temperature in the targeted tissues. This can be done with an invasive thermocouple that is inserted into the tissue, but that defeats the purpose of having a truly non-invasive treatment. That's why our lab and others turn to magnetic resonance imaging (MRI) as a non-invasive technology for measuring temperature changes.
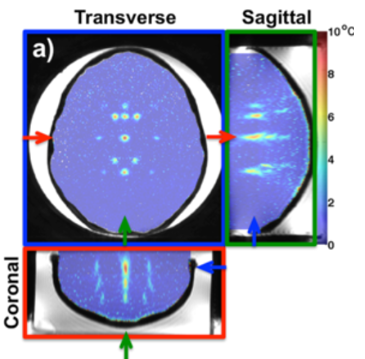
|
Several parameters used in MRI are sensitive to temperature changes, including the proton density, the T1 and T2 relaxation times, the diffusion coefficient, and the proton resonance frequency (PRF). The PRF method is most commonly used to measure temperatures within aqueous tissues. It works by measuring a phase shift between two MRI images, which results from the temperature-dependent frequency of hydrogen protons in water. The measured phase change is linearly correlated with the change in temperature. Our lab has developed several techniques that use the PRF method to volumetrically measure temperatures in real time.
The lack of hydrogen bonding within fat-based tissues renders the PRF method very insensitive. However, there is a strong clinical need to monitor temperature in fat for FUS treatments in areas such as the breast and abdomen. Our lab has demonstrated that the MRI relaxation time T1 for human tissue also demonstrates a linear relationship with temperature. By analyzing the T1 relaxation time within tissues, not only can a temperature map of the tissue be accurately quantified, but the damage to the tissue as a result of the heating can be determined as well. Our lab seeks to improve this technique by increasing the temporal and spatial resolution of these temperature measurements and increasing the field of view to allow for quantification of the fully isonified field of view.
Related Publications
Odéen H. and Parker DL (2019). Magnetic resonance thermometry and its biological applications - Physical principles and practical considerations. Progress in NMR Spectroscopy. 110:34-61.
Diakite, M., Odéen, H., Todd, N., Payne, A., & Parker, D. L. Toward real‐time temperature monitoring in fat and aqueous tissue during magnetic resonance–guided high‐intensity focused ultrasound using a three‐dimensional proton resonance frequency T1 method .Magn Reson Med. 2014;72(1):178-187.
Diakite M, Payne A, Todd N, Parker DL. Irreversible change in the T1 temperature dependence with thermal dose using the proton resonance frequency-T1 technique .Magn Reson Med. 2013;69(4):1122-30.
Odeen H, Almquist S, de Bever J, Christensen DA, Parker DL. MR thermometry for focused ultrasound monitoring utilizing model predictive filtering and ultrasound beam modeling .Journal of therapeutic ultrasound. 2016;4:23.
Odeen H, de Bever J, Almquist S, Farrer A, Todd N, Payne A, et al. Treatment envelope evaluation in transcranial magnetic resonance-guided focused ultrasound utilizing 3D MR thermometry. Journal of therapeutic ultrasound. 2014;2:19.
Svedin BT, Payne A, Bolster BD, Jr., Parker DL. Multiecho pseudo-golden angle stack of stars thermometry with high spatial and temporal resolution using k-space weighted image contrast. Magn Reson Med. 2018;79(3):1407-19.
Todd N, Adluru G, Payne A, DiBella EV, Parker D (2009). Temporally constrained reconstruction applied to MRI temperature data. Magn Reson Med. 62(2), 406-19.
Todd N, Payne A, Parker DL (2010). Model predictive filtering for improved temporal resolution in MRI temperature imaging. Magn Reson Med. 63(5), 1269-79.
Todd N, Vyas U, de Bever J, Payne A, Parker DL (2011). The effects of spatial sampling choices on MR temperature measurements. Magn Reson Med. 65(2), 515-21.
Todd N, Vyas U, de Bever J, Payne A, Parker DL (2012). Reconstruction of fully three-dimensional high spatial and temporal resolution MR temperature maps for retrospective applications. Magn Reson Med. 67(3), 724-30.
Todd N, Diakite M, Payne A, Parker DL (2013). Hybrid proton resonance frequency/T1 technique for simultaneous temperature monitoring in adipose and aqueous tissues. Magn Reson Med. 69(1), 62-70.
Odéen H and Parker DL (2019). Improved MR thermometry for laser interstitial thermotherapy. Lasers Surg. Med. (Early view).
Members of the Focused Ultrasound Laboratory have intellectual property in this area of research. Any conflict of interest concerns can be addressed by the investigators or the department chair.
