Welcome!
Welcome!
The Baker Lab has two major areas of investigation. The first is understanding lifespan evolution between species. The lifespans of organisms show a tremendous degree of variance, even among vertebrates. The pigmy goby, Eviota sigillata, has a maximum lifespan of 60 days, whereas the Greenland shark, Somniosus microcephalus, has been estimated to live up to 4 centuries. In animals housed under optimal conditions, with unlimited access to food, shelter from the elements, protection from predation, and access to veterinary care, the degree of variation in lifespan between species far outweighs the degree of variation within species. Given that environmental differences are minimized under these circumstances, this suggests that the major determinants of lifespan variation between species are genetic. Yet, the underlying genes and pathways that control lifespan between species are poorly understood. We are developing computational and biochemical tools to uncover the mechanisms resulting in the vast differences in lifespan observed in nature.
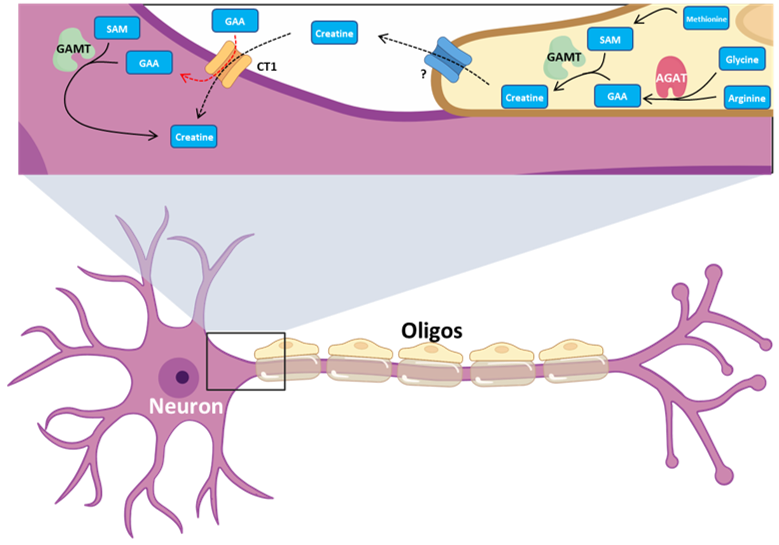
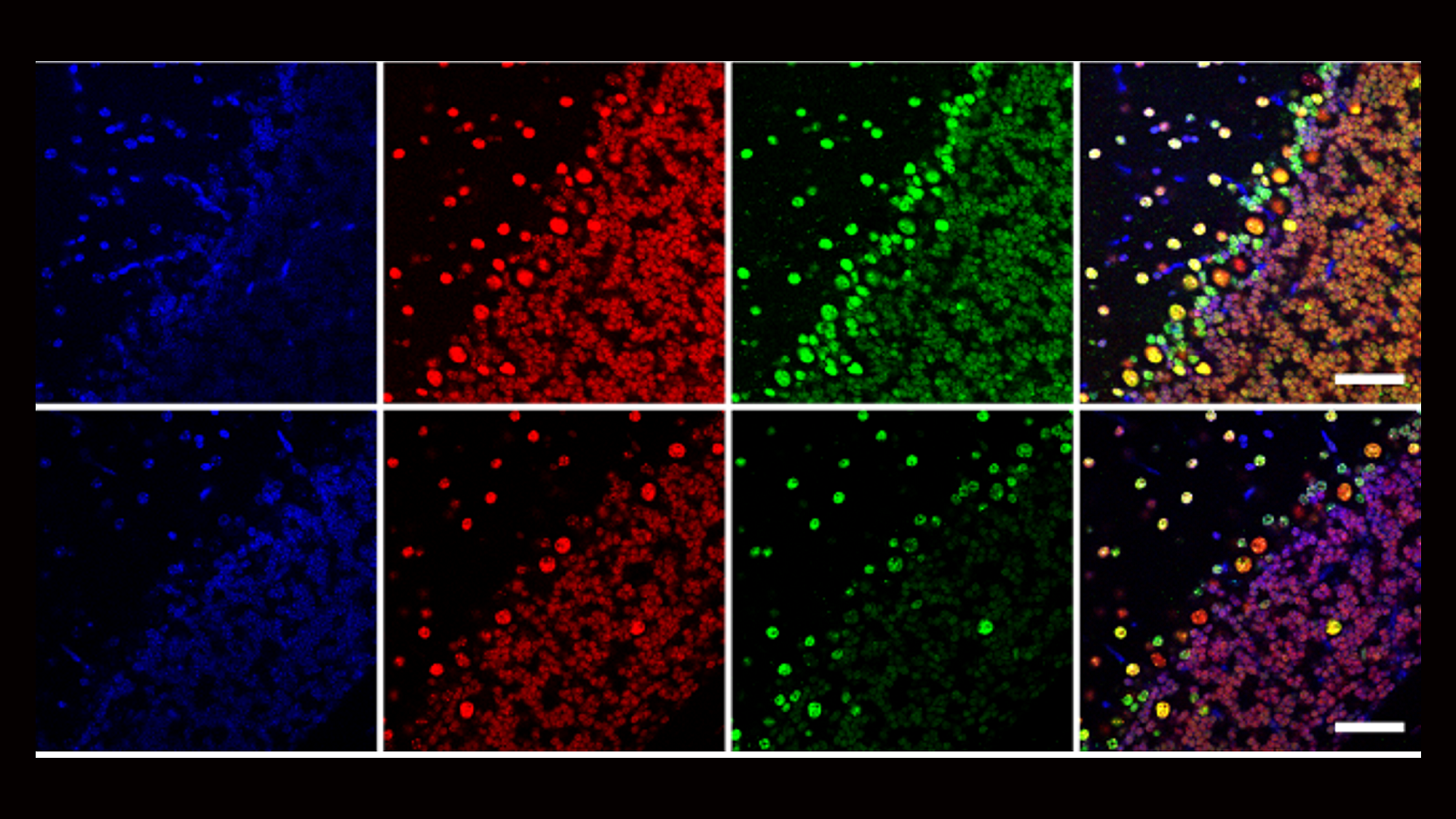
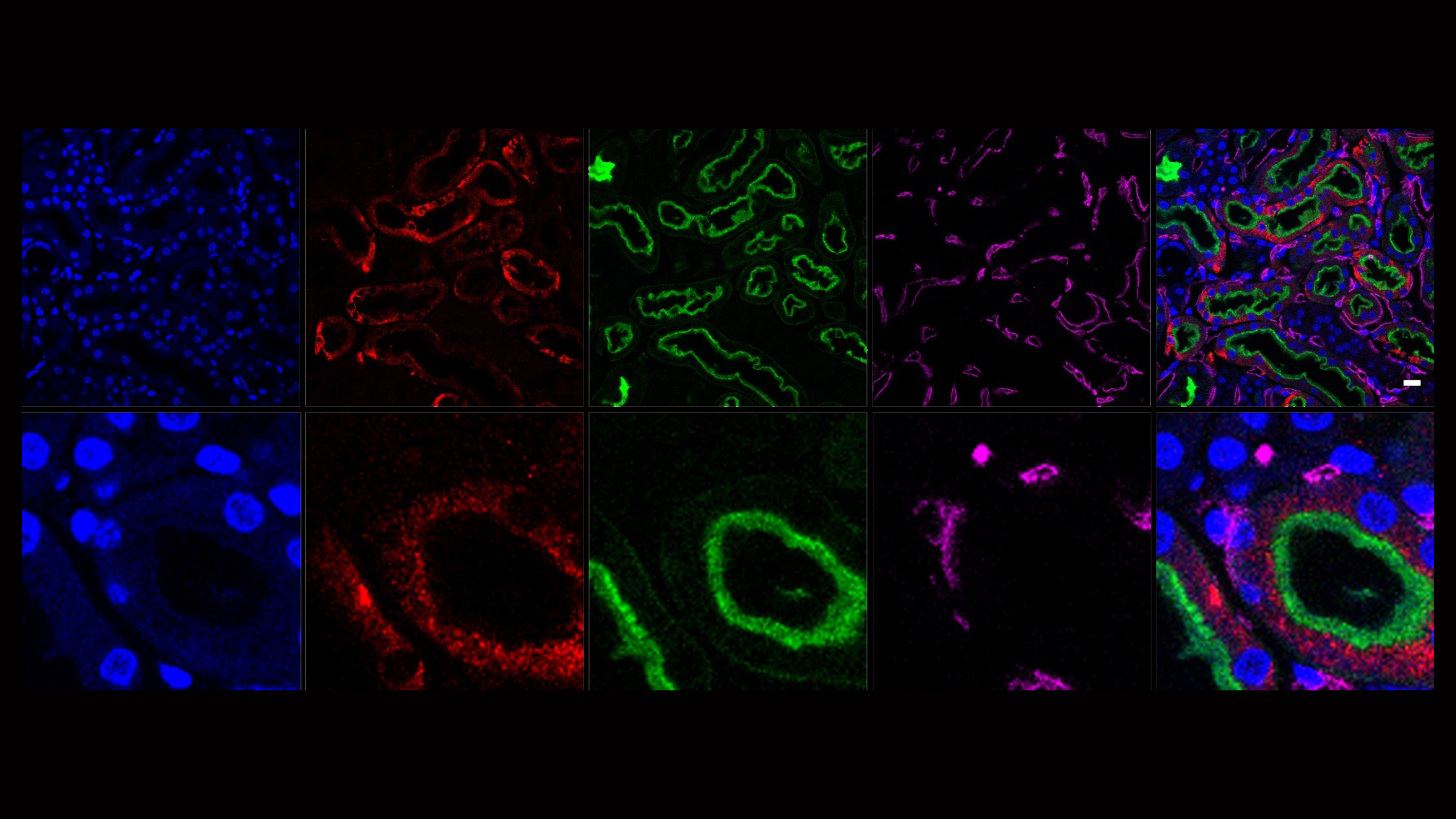
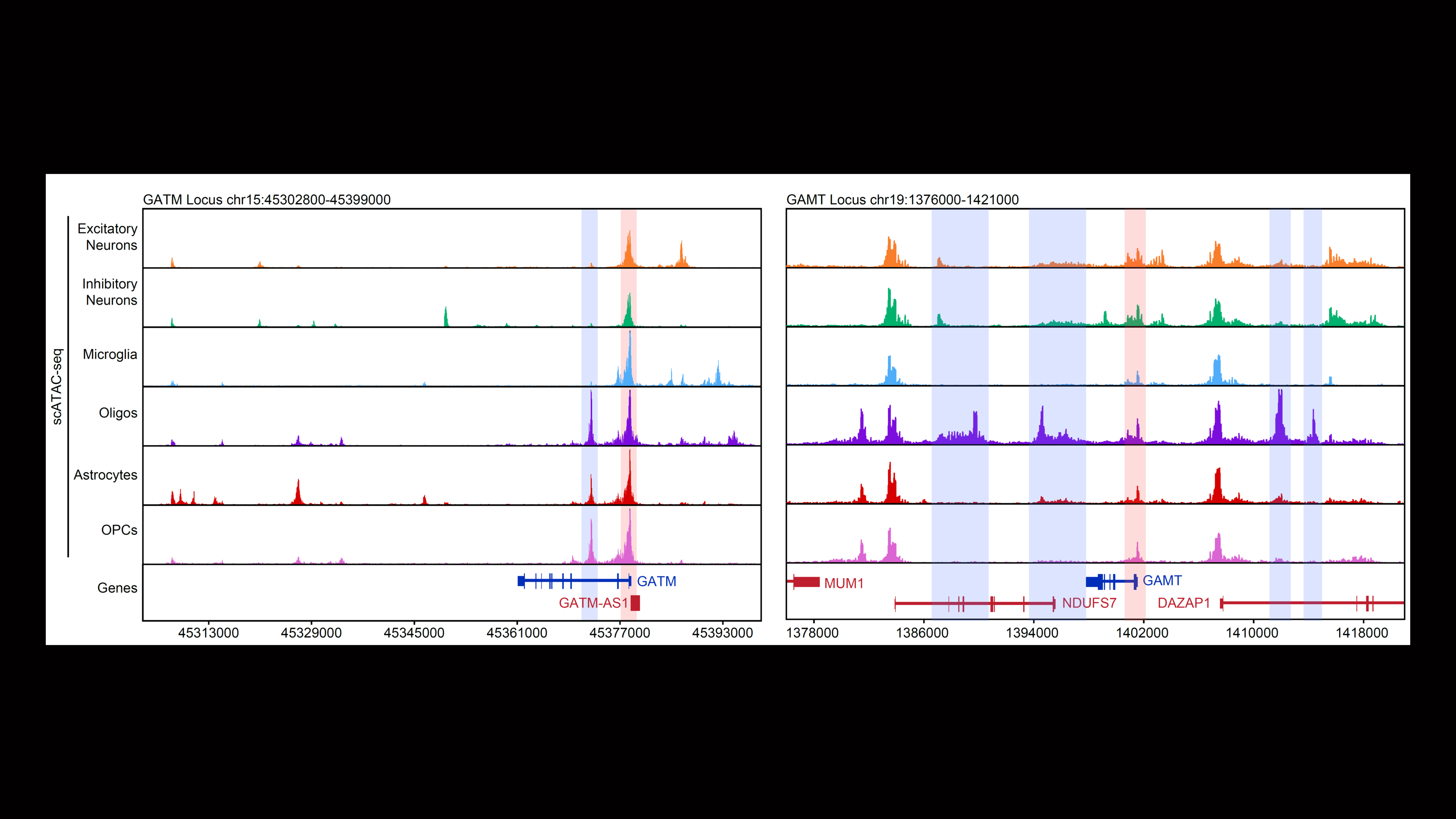
Our second major area of research involves work on Cerebral Creatine Deficiency Syndromes (CCDSs). Defects in creatine metabolism result in a devastating neurological syndrome affecting pediatric patients. Unfortunately, the predominant form of CCDS (type I or Creatine Transporter Deficiency, CTD) currently has no effective treatment options. We are currently using a combination of single-cell RNA sequencing (scRNA-seq), scATAC-seq, and high resolution microscopy to develop treatment strategies for this and related disorders.

Steve Baker, M.D. Ph.D.
Assistant Professor in the Department of Pathology, Principal Investigator
Dr. Baker is a physician-scientist who currently serves as an Associate Director of Transfusion Medicine with University of Utah Health. His scientific interests span a wide variety of topics, from pediatric neuroscience to age-related disease. He is currently funded by an award from the Association for Creatine Deficiencies (ACD) to investigate a potential gene therapy for patients with Creatine Transporter Deficiency. He is also developing computational tools to study vertebrate lifespan variation.
Email Address: stevenDOTbakerATpathDOTutahDOTedu

Sadie Johnson, B.A.
Sadie joined the Baker Lab after completing her undergraduate work at Utah Valley University
majoring in Psychology with a minor in Biology. She is currently researching how a recurrent substitution found in long-lived species modulates electron transport chain maturation. She plans to continue her career by applying to M.D./Ph.D. programs.

Tarun Yadav, B.S. M.S.
Tarun joined our group after completing his undergraduate studies at the Indian Institute of Science Education and Research, Pune specializing in Biology. He is currently investigating mitochondrial protein evolution. Human mitochondria contain approximately 1,500 different proteins of which the vast majority are encoded by the nuclear genome. A much smaller set (13 protein coding genes) are expressed from the mitochondrial genome in essentially all metazoans. Tarun’s project is investigating how genes that were once encoded by the mitochondrial genome have evolved to be expressed from the nucleus.

Chloe Wells, B.S.
Chloe joined the Baker Lab after completing her undergraduate studies at Indiana University majoring in Neuroscience. She will testing a gene therapy for Creatine Transporter Deficiency using primary cells and mouse models. She hopes to apply for Veterinary School in the coming future.

Jon Sorgenfrei B.S.
Jon joined the Baker Lab having finished his undergraduate studies at Ball State University double majoring in Psychological Science and Sociology. He then worked in the clinical laboratory for a year with Quest Diagnostics, developing expertise in chemistry, hematology, and infectious disease testing prior to joining our group. He will be carrying out studies on patient derived fibroblasts from children with Creatine Transporter Deficiency. He plans to apply to graduate school and obtain a Ph.D. in neuroscience or a related field.

Devin R. Albertson, B.S.
Devin joined the Baker Lab after finishing her undergraduate studies at Clemson University majoring in Microbiology with minors in Sociology and Biological Sciences. She then worked for one year as a Lab Associate in the Cytopathology Laboratory at the Medical University of South Carolina. She will be developing skills in computation and performing bench experiments on lifespan associated variants. She plans to apply for a spot in a program offering a Masters of Public Health.
Comparative Genomics of Lifespan
Learn more about our work using genomic technologies to understand lifespan evolution in vertebrates.
Cerebral Creatine Deficiency Syndromes
Learn more about our work to understand creatine metabolism and develop treatment approaches for patients with genetically caused creatine deficiency.
Selected Publications
Baker SA and Rutter J. Metabolites as signaling molecules. Nat Rev Mol Cell Biol. 2023 Jan 12. doi: 10.1038/s41580-022-00572-w. PMID: 36635456
Baker SA, Wong LK, Wieland R, Bulterys P, Allard L, Nguyen L, Quach T, Nguyen A, Chaesuh E, Cheng P, Bowen R, Virk M. Validated transport conditions maintain the quality of washed red blood cells. Transfusion. 2022 Sep;62(9):1860-1870. PMID: 36084205
Baker SA, Gajera CR, Wawro AM, Corces MR, Montine TJ. GATM and GAMT synthesize creatine locally throughout the mammalian body and within oligodendrocytes of the brain. Brain Res. 2021 Nov 1;1770:147627. PMID: 34418357
Wawro AM, Gajera CR, Baker SA, Nirschl, JJ, Vogel H, Montine TJ. Creatine transport and pathological changes in creatine transporter deficient mice. J Inherit Metab Dis. 2021 Jan 2. PMID: 33389772
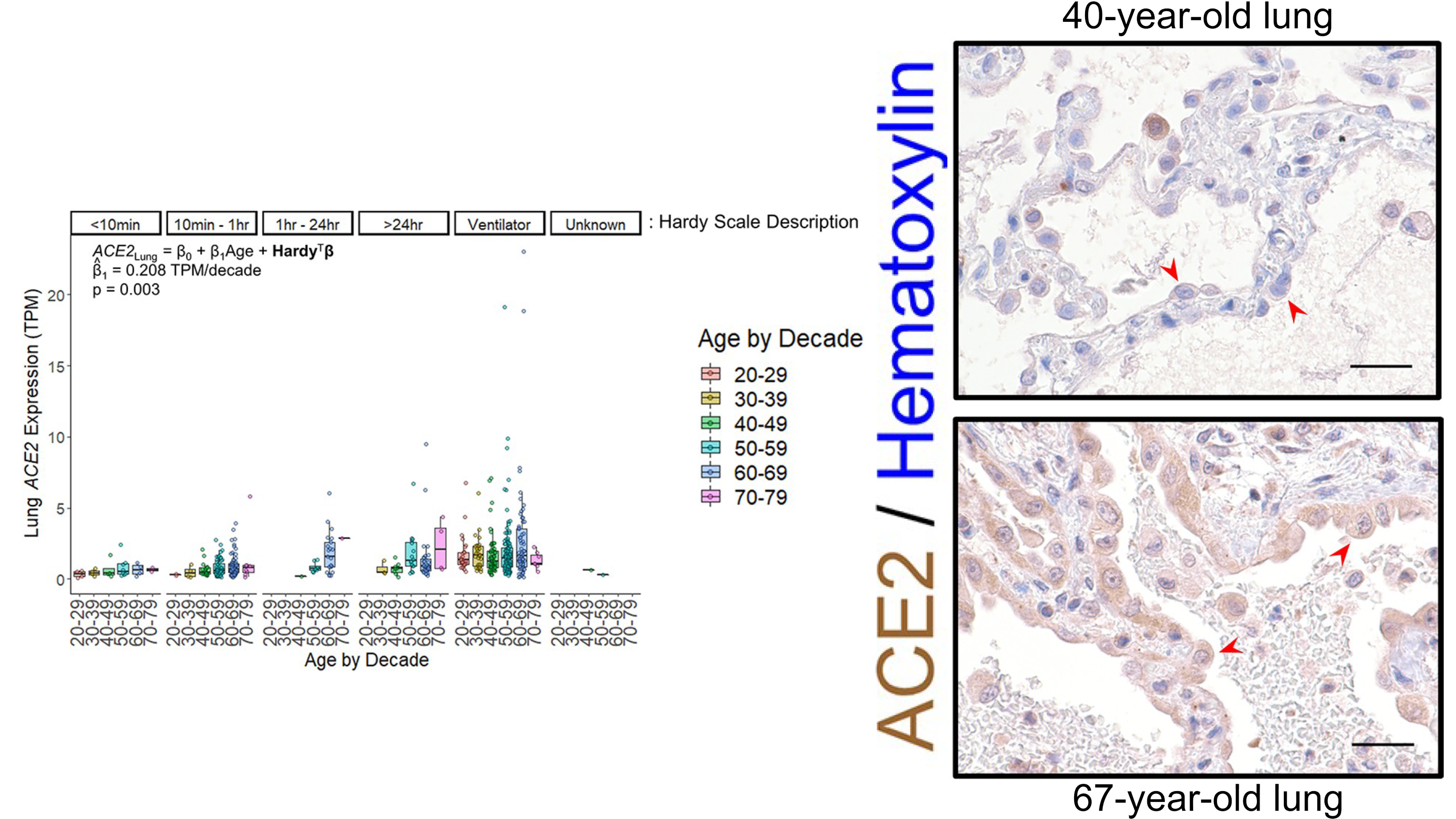
Baker SA, Kwok S, Berry GJ, Montine TJ. Angiotensin-converting enzyme 2 (ACE2) expression increases with age in patients requiring mechanical ventilation. PLoS One. 2021 Feb 16;16(2):e0247060. PMID: 33592054
Baker SA, Jin J, Pfaffroth C, Vu T, Zehnder JL. DOAC-Stop permits the safe and effective removal of direct oral anticoagulant interference in lupus anticoagulant testing. Res Pract Thromb Haemost. 2021. Jan 27;5(2):314-325. PMID: 33733031
Ito-Ishida A, Yamalanchili HK, Shao Y, Baker SA, Heckman LD, Lavery LA, Kim JY, Lombardi LM, Sun Y, Liu Z, Zoghbi HY. Genome-wide distribution of linker histone H1.0 is independent of MeCP2. Nat Neurosci. 2018 Jun;21(6):794-798. PMID: 29802390
Lombardi LM, Zaghlula M, Sztainberg Y, Baker SA, Klisch TJ, Tang AA, Huang EJ, Zoghbi HY. An RNA interference screen identifies druggable regulators of MeCP2 stability. Sci Transl Med. 2017 Aug 23;9(404). pii: eaaf7588 PMID: 28835516
Goodnough L, Baker S, Shah N. How I Use Clinical Decision Support to Improve Red Blood Cell Utilization. Transfusion. 2016. Oct;56(10):2406-2411. PMID: 27546388
Baker S*, Lombardi L*, Zoghbi H. Karyopherin Alpha 3 and Karyopherin Alpha 4 Proteins Mediate Nuclear Import of MeCP2. J Biol Chem. 2015 Sep 11;290(37):22485-93. PMID: 26245896 *equal contribution
Baker S, Chen L, Wilkins A, Yu P, Lichtarge O, Zoghbi H. An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders. Cell. 2013 Feb 28;152(5):984-96. PMID: 23452848
Welcome New Lab Members
Welcome to the following new members of our lab!
Chloe Wells, Jon Sorgenfrei, and Devin Albertson.
We are excited to have you on the team!
Join Us!
Are you looking to make a difference! If you are looking to join a research team that is looking to make a difference in the world, contact us.