Projects

Projects
TUNEL Assay
Research Project
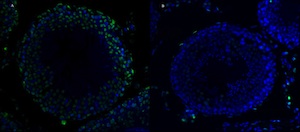
These photos show DNA fragmentation in rat testis tissue sections by TUNEL assay. The TUNEL positive cells stain green and the counter stain is blue (Hoechst). Photo A shows the positive control of TUNEL assay: the testis tissue section has been treated with DNase I to fragment DNA. Photo B shows TUNEL assay of the normal rat testis tissue section.
Topoisomerase
Research Project
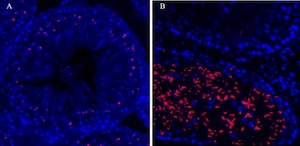
These photos show topoisomerase IIα (A) and topoisomerase IIß (B) positive cells in rat testis tissue sections. The topoisomerase IIα shows in spermatocytes and the topoisomerase IIß shows in round spermatids.
Topo and TUNEL
Research Project
This photo shows the rat seminiferous tubules. The blue shows the cell nuclears stained by Hoechst. The green shows the DNA fragmentation stained by TUNEL assay. The red demonstrates the topoisomerase II beta by immunofluroscence.
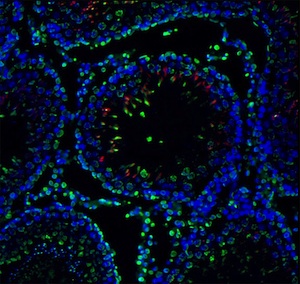
Cell Lineage Segregation in Early Embryos
Research Project
During pre-implantation embryo development, the first morphologically distinguishable cell types are the differentiated trophectoderm (TE) and the pluripotent inner cell mass (ICM) at blastocyst stage. These two cell types have distinct fates; the TE will form the embryonic portion of the placenta, while the ICM will form the future embryo and other extraembryonic tissues. Understanding the regulatory mechanisms underlying cell differentiation in the early embryo is still unclear. The relationship of the expression of different transcription factors that are associated with each cell type will likely help us understand the first cell lineage decision. Currently in our laboratory we are investigating the expression pattern of these genes in single mouse blastomeres of different developmental stages. It is believed that insight into the molecular mechanisms of cell lineage establishment in preimplantation embryo could aid in developing more suitable culture systems for embryos, and in better understanding causes of poor embryo development.
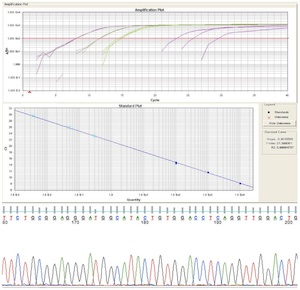
Genetic Studies
Research Project
Several causes of male infertility, including endocrine disorders, varicoceles, developmental abnormalities and microdeletions of the Y chromosome have been identified. However, the majority of male infertility remains unexplained. It is estimated that more than 2,500 genes play a role in spermatogenesis, and it is likely that mutations or abnormalities in the regulation of these genes are responsible for the unexplained infertility experienced by many patients. To determine which genes may carry mutations associated with infertility, we have collected DNA from a panel of severely infertile men and are in the process of sequencing specific genes, looking for mutations that may cause infertility. We have completed the sequencing of several genes already and have many ongoing projects.
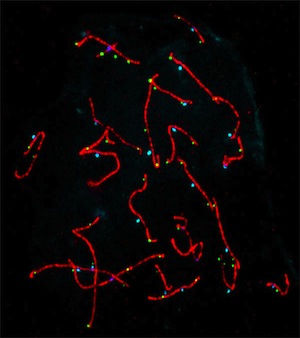
Meiotic Errors
Research Project
During the process of meiosis, chromosomes separate and segregate into different cells that will eventually mature into either sperm in males or eggs in females. Errors in meiosis result in an abnormal number of chromosomes in a cell and are a leading cause of infertility. Meiotic errors are also responsible for birth defects such as Down Syndrome and Turner Syndrome. In order to study meiotic errors it is necessary to look at sperm during an early state of development known as the pachytene stage. The pachytene cells are collected from testicular tissue and placed on a glass slide where they undergo procedures that release the chromosomes from the cell, making the individual chromosomes visible. By using fluorescent probes and antibodies, we can study both the rate of meiotic errors and how the proteins responsible for meiosis are altered in different populations of men.
Protamines
Research Project
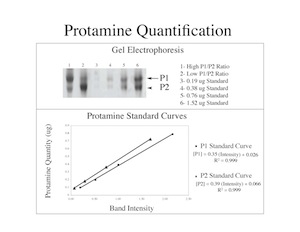
The protamines, protamine 1 (P1) and protamine 2 (P2), are sperm specific nuclear proteins. During spermiogenesis, protamines are used to package the sperm DNA in a highly condensed form. A deficiency of sperm protamines is associated with human male infertility. Our research shows that abnormal sperm protamine levels are a common defect in infertile patients, but not in donors of known fertility. We have identified abnormal ratios of protamines, correlating with infertility, in several different populations of patients. In addition, we’ve demonstrated that the DNA in sperm with abnormal levels of ratios of protamines is susceptible to damage. Currently, we are investigating the control of protamine expression, in both patients with normal and abnormal protamine levels. Our goal is to identify the interactions associated with transcription and translation that control the expression of P1 and P2 and the events that lead to deregulation of these proteins.
Modeling Infertility In Animals
Research Project
A useful tool in determining a gene’s function is developing an animal model in which the gene has been disrupted. The deficiencies observed in the experimental animal generated, typically a mouse, can then be attributed to a lack of the gene product. One of our research goals is to develop new strategies to disrupt specific genes important for spermatogenesis. RNA interference (RNAi) is a relatively new technique that prevents the messenger RNA of a gene from being translated into a protein. We are currently working on a way to use RNAi, in combination with a novel delivery system, to silence genes only in the testis. This research may lead to more rapid and cost effective ways to determine the function of genes related to spermatogenesis.

Varicoceles
Research Project
A varicocele is a swelling of the spermatic veins, they are present in approximately 15% of the general population and 25% of men with infertility. A debate has existed for years about if or how they affect infertility, and whether surgically removing the varicocele will benefit the patient. To help answer these questions we have worked with the institutional animal use committee (IACUC) to develop surgically induced varicoceles in rates and are in the process of collecting data on how the varicoceles influence sperm production both in general and in the presence of different toxic agents such as lead and nicotine.
Oocyte In Vitro Growth and Maturation
Research Project
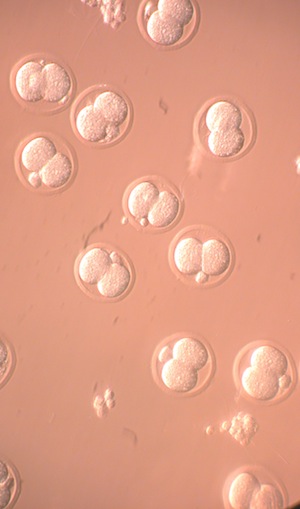
Between birth and the onset of puberty, the number of follicles in the human ovaries drops from two million to approximately 500,000. Each month after puberty, a large batch of follicles, with the exception of one that is ovulated, degenerate, further reducing the pool of reserve follicles available. Advancing age, cancer treatments and other procedures can also rapidly reduce or even eliminate the ovaries store of follicles that could produce an egg. as a result, many women, due to age or cancer are unable to conceive. While ovarian tissue containing the immature follicles and eggs can be cryopreserved and stored for later use, it is still necessary to develop protocols to mature the egg-containing follicle so that a mature egg can be harvested and used for reproduction. We are currently working on developing in vitro maturation protocols, using a mouse model, that will eventually help us develop healthy fertile eggs for patients. Some of the topics we are studying include: in vitro oocyte growth from pre-antral follicles, in vitro oocyte maturation and fertilization and cytogenetic changes in oocytes and embryos derived from in vitro maturation.
Human In Vitro Fertilization Studies
Research Project
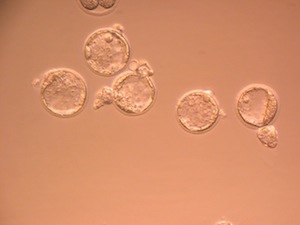
Thousands of couples undergo in vitro fertilization (IVF) every year in an effort to become pregnant. As an academic care center, on of our goals is to improve the quality of care IVF patients receive by conducting research that will result in better treatments and higher pregnancy rates Some of our recent and ongoing studies include comparisons of different embryo culture media, how varying concentrations of gasses in the atmosphere affect embryo development, and how a patient’s body mass index (BMI) correlates with IVF outcome. We are also actively investigating new ways to assess which patients will benefit from different forms of treatment, based on results from diagnostic tests like the sperm penetration assay.
General Population Studies
Research Project
An important aspect of research is understanding how a study population differs from the general population. Currently, there is only limited data on the semen parameters of the general population and how they relate to various environmental and genetic factors. As a resource for our own research and investigators world wide, we are recruiting males from the general population and collecting data on fertility and semen parameters. Besides a semen analysis and hormone profile, each participant fills out an extensive medical history and questionnaire that covers multiple topics including use of dietary supplements, activity levels, occupations, environmental exposures, and genetic factors. Semen and blood samples are also collected and stored from each participant to provide DNA and study samples for future research projects.