Test Report Examples

Test Report Examples
Immunodermatology Laboratory Test Reports
Immunodermatology Laboratory Test Reports are organized to provide tiered information starting with the abstracted Diagnostic Interpretation (critical for clinical relevance), followed by the data obtained from the testing in Results, and then Comments regarding the specific findings and the testing generally with Testing Methods at the report end. Charts, Graphs, or Images may follow for additional information.
The Clinical Information provided by the ordering Clinician is necessary for a relevant interpretation and pertinent comments including further testing recommendations, if indicated.
Serum Test Report Guide
Specimen Details
_________________________________________________
This includes specimen identification with collected and
received dates and ordered testing.
_________________________________________________
Clinical/Diagnostic Information
This content is provided by the ordering clinician and
includes the reason for testing. (Note: Clinical information is
critical for accurate and relevant interpretation and comments.)
DIAGNOSTIC INTERPRETATION
This is a synopsis of key findings from the testing and
their diagnostic relevance.
_________________________________________________
RESULTS
This section reports the discrete finding and value of each
test component, along with the reference range.
_________________________________________________
COMMENTS (Specific)
These comments provide an explanation of the test results
as they relate to clinical considerations and include
reference to any concurrent and/or previous testing.
ELISA RESULTS GRAPH
------------------------------------------------------------
A trend graph of ELISA results is included if previous and/or
concurrent testing has been performed demonstrating increased
antibody levels; the graph may be found on a subsequent page.
TEST RESULTS SUMMARY CHART
------------------------------------------------------------
A chart tabulating results of tests is included if previous
and/or concurrent testing has been performed.
COMMENTS (General)
These comments summarize information about the test(s)
performed and the component(s) assessed to aid in
interpretation of their clinical applicability.
_________________________________________________
TESTING METHODS
The section lists the procedures performed, the test
source(s), and the applicable laboratory-developed test
disclaimer(s).
Tissue Biopsy Test Report Guide
Specimen Details
_________________________________________________
This includes specimen identification with collected and received
dates.
_________________________________________________
Specimen(s)
This is a description of body location and whether it is
lesional or perilesional for each specimen. (Note: Biopsy site
and adequate tissue are critical for accurate diagnostic findings.)
Clinical/Diagnostic Information
This content is provided by the ordering clinician and
includes the reason for testing.
DIAGNOSTIC INTERPRETATION
This is a synopsis of key findings from the testing and
their diagnostic relevance.
_________________________________________________
RESULTS
This section reports the discrete finding for each test
component on each specimen, which, for direct
immunofluorescence, includes IgG, IgG4, IgM, IgA, third
component of complement (C3), and fibrinogen.
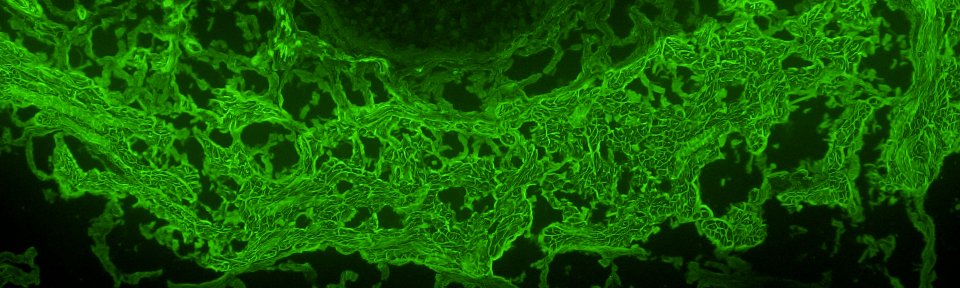
RESULT IMAGES (only included and available when applicable)
Images of unusual, select positive, and/or special interest findings* are displayed in this section and may be found on the subsequent page. (High resolution, color digital files of the images may be requested by contacting the University of Utah Immunodermatology Laboratory.)
* Note that immunofluorescence-stained tissue slides are not kept longer than two weeks after testing because fluorescence fades and patterns become indiscernible; therefore, additional images cannot be acquired after that time without retesting.
_________________________________________________
COMMENTS
The comments provide an explanation of the test results as
they relate to clinical considerations and may include
recommendations to correlate with other testing, including
serum testing, to enhance diagnostic sensitivity.
_________________________________________________
TESTING METHODS
The section summarizes the procedures performed, the
interpretation schema, and the applicable laboratory-developed
test disclaimer.
Serum Test Report Examples
•Immunobullous Disease Antibody Panel
•Basement Membrane Zone Antibody Panel
•Pemphigus Antibody Panel, IgG
Tissue Biopsy Test Report Examples
•Direct Immunofluorescence
•Eosinophil Granule Major Basic Protein 1
Board Certified
John J. Zone, M.D., Kristin M. Leiferman, M.D., and Melanie K. Kuechle, M.D. are American Board of Dermatology Diplomats in Dermatology and Dermatological Immunology / Diagnostic and Laboratory Immunology. Mazdak A. Khalighi, M.D. and Margaret M. Cocks, Ph.D., M.D. are American Board of Pathology Diplomats in Anatomic Pathology. Dr. Khalighi additionally has fellowship training and faculty experience in Renal Pathology/Immunopathology. Dr. Cocks additionally is an American Board of Pathology Diplomat in Clinical Pathology and is an American Board of Dermatology Diplomat in Dermatology and Dermatopathology. Drs. Khalighi and Cocks have had specialized, fellowship-equivalent training in laboratory immunodermatology by Drs. Zone and Leiferman.
Contact Us
Immunodermatology Laboratory,
Department of Dermatology
University of Utah Health
417 South Wakara Way, Suite 2151
Salt Lake City, UT 84108