Research
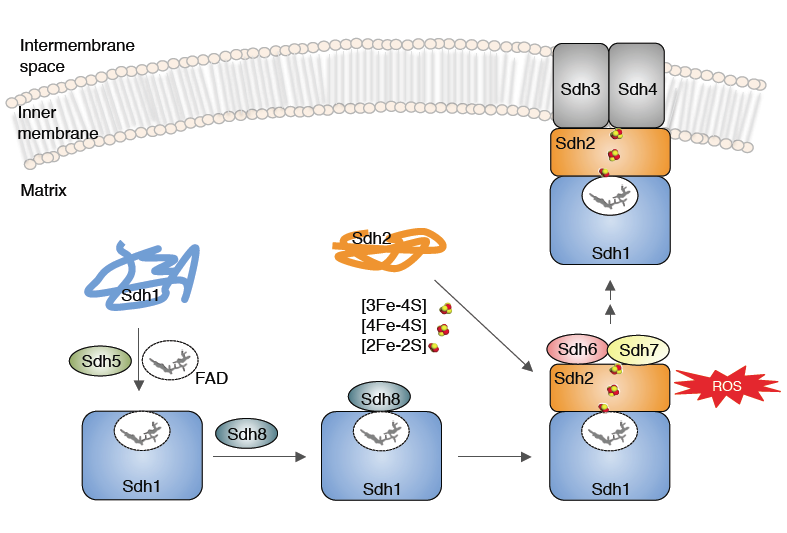
Figure Legend: Model of the role of Sdh6(SDHAF1) and Sdh7(SDHAF3) in maturation of Sdh2(SDHB). Sdh6 and Sdh7 associate with Sdh2 within a Sdh1/Sdh2 intermediate. Sdh1 maturation requires covalent flavinylation by Sdh5 followed by formation of the Sdh1/Sdh2 subcomplex that is chaperoned by Sdh8.
Defects in the assembly of the mitochondrial OXPHOS respiratory chain contribute to numerous inherited and acquired diseases of patients with cardiomyopathy, hepatopathy, and neurological disorders. We are interested in elucidating the mechanisms of assembly of respiratory complexes II, III and IV in yeast as an experimental system. Many assembly factors are known that mediate assembly of these respiratory complexes and a large fraction of these are conserved in human cells. Thus, functional studies in yeast contribute to our understanding of the assembly processes in humans. We seek to identify new proteins that mediate the biogenesis of the OXPHOS respiratory complexes and elucidate their mechanism of action. One major focus concerns the assembly of flavin, heme and metal cofactor sites in the respiratory complexes.
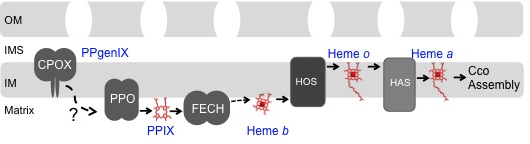
The assembly of CIV cytochrome oxidase (CcO) requires the coordinate assembly of subunits translated in both the cytoplasm and mitochondria as well as the insertion of heme and copper redox cofactors. CcO biogenesis commences with the synthesis of Cox1 followed by formation of the heme a cofactor centers and subsequent addition of other subunits. The Cox1 assembly intermediate is conditionally deleterious in cells stalled in subsequent assembly steps. The high spin heme a3 center in Cox1 can generate reactive oxygen species when the Cox1 intermediate accumulates. To avoid the potential danger of this pro-oxidant intermediate, yeast employ chaperone proteins to guide this assembly intermediate through the Cox1 maturation process and induce a quality control protease to remove any stalled assembly intermediates. We are focused on elucidating the process of redox cofactor insertion as well as the quality control proteolytic system. We seek to identify additional assembly factors in the CcO biogenesis process and elucidate the mechanism of their assembly function. In these studies, we use a combination of in vitro biochemical, in vivo cellular assays and genetic analyses to elucidate the mechanism and pathway by which these assembly proteins mediate CcO formation.
Succinate dehydrogenase (Complex II) and cytochrome c reductase (Complex III) also contain a myriad of metal cofactors including FeS and heme centers. We seek to understand the assembly of these centers. We identified a new Complex III assembly factor Mzm1 that functions in a late step of CIII assembly in mediating insertion of the Rieske Fe/S subunit in conjunction with the Bcs1 AAA ATPase.
Succinate dehydrogenase (CII) contains a covalently bound FAD, 3 distinct Fe/S centers and a membrane associated heme moiety. The SDH structure and its cofactors is shown below. We identified a new SDH assembly factor (Sdh7) and demonstrated that Sdh7 functions in conjunction with Sdh6 in the maturation of the FeS cluster subunit Sdh2. Since mutations in one SDH assembly factor predisposes patients to tumors, the identification of additional SDH assembly factors may be associated with idiopathic SDH-associated diseases.
We are studying the pathway of FeS cluster insertion into Sdh2. FeS cluster biogenesis occurs in the mitochondrial matrix and pre-assembled clusters are transferred to client proteins in a protein-mediated manner.
We are also interested in the routing of the heme cofactor from its site of synthesis on ferrochelatase to client proteins both in the mitochondrial matrix and intermembrane space. Little is known about heme trafficking in eukaryotic cells.