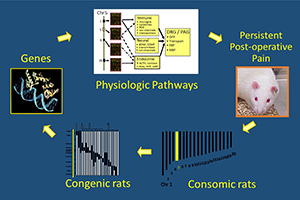
Chronic (or persistent) postoperative pain (CPOP) is a potentially devastating outcome from an otherwise successful surgical procedure. It affects millions of patients every year, with pain lasting for months to years, resulting in patient suffering and resulting economic hardship. The surgeries with the highest incidence of chronic postoperative pain are amputations, thoracotomies, cardiac, and breast surgery. Other risk factors include preoperative pain, psychological factors, demographics, and the intensity of acute postoperative pain. Attempts to prevent chronic postoperative pain have largely been unsuccessful, with no change in the incidence despite increased use of regional and multimodal analgesia. Therefore, further research is needed to identify biomarkers to accurately predict those at risk for developing chronic postoperative pain and treatments that reduce the incidence. We hypothesize that Diffuse Noxious Inhibitory Control (DNIC) efficiency is predictive of who will develop chronic postoperative pain. Thus, a better understanding of the mechanisms responsible for DNIC will result in more efficacious treatments. We would expect that patients or animal models with less efficient DNIC would be ‘at risk’ for developing chronic pain when exposed to the painful stimulus of surgery. Our overall objectives in this application are to use a new model of persistent postoperative pain, the Dahl S rat, to investigate the involvement of serotonin, catecholamine and dopamine systems on DNIC using pharmacologic, chemogenetic and optogenetic approaches. We will also investigate which genetic polymorphism(s) are responsible for the persistent postoperative pain experienced by the Dahl S rat. This will be accomplished in three projects. Project 1: will determine the relationship between DNIC and CPOP. DNIC responses will be abolished in Sprague Dawley rats and restored in Dahl S rats, and the resultant effects on postoperative pain persistence ascertained. We will also test the hypothesis that the absent DNIC response in SS rats is a result of increased nociceptive facilitation by serotonergic “on cells” in the rostral ventral medulla by optogenetically inhibiting serotonergic neurons in the spinal cord. Project 2 will examine the role of periaqueductal gray dopamine neurons on DNIC and postoperative pain using a Dahl S rat expressing a novel variant of the Catecholamine-O-methyltransferase gene that increases dopaminergic tone. Project 3 will use a powerful physiologic genomics approach, the use of consomic and congenic rats, to identify the gene polymorphism(s) responsible for the absent DNIC response and persistent postoperative pain exhibited by Dahl S rats. We expect our studies to provide genetic and phenotypic biomarkers to guide diagnosis and treatment decisions in chronic postoperative pain.
Dr. Taylor’s project, funded for $1.9 million will start August 1st, 2020 and commence on July 31, 2024.