 |
| Department of Radiology and Imaging Sciences faculty member and UCAIR researcher, Jeffrey S. Anderson, MD, PhD. |
By Michael Mozdy
Imagine you’ve just been told by your child’s pediatrician that your two-year-old is likely “on the spectrum.” She’s referring to autism spectrum disorder (ASD), and after the shock, you have a lot of questions. Frustratingly, most of the answers include a form of “each child with autism is different” or “we’ll have to wait to see.” While behavioral interventions can be very successful in preparing children with autism to live in today’s society, it takes years of hard work and, often, much trial and error.
You, like the parents of an estimated 15,000 children in Utah1, are left wondering why there isn’t a better way to know how to help your child right now.
Thankfully, several researchers at the University of Utah are working on a better way. Jeffrey S. Anderson, MD, PhD, is one of them. A faculty member of the Department of Radiology and Imaging Sciences, he is applying his state-of-the-art MRI imaging expertise to the brains of people with autism to create a much more comprehensive and nuanced method for diagnosis and treatment of ASD.
The Many Modes of MRI
Anderson’s team uses magnetic resonance imaging (MRI) to examine and map the brains of people with autism. Various MRI techniques provide clear pictures of brain anatomy. Structural MRI reveals “grey matter,” which occurs in the outer portion of the brain and contains mostly nerve cells (neurons). Diffusion tensor MRI, on the other hand, is excellent at revealing “white matter.” White matter is found in the deeper tissue of the brain and is made up of nerve fibers, or axons, which branch out from and carry signals to the neurons. In other words, white matter is the massive, interconnected superhighway that carries brain signals, and MRI gives Anderson a detailed look at this infrastructure.
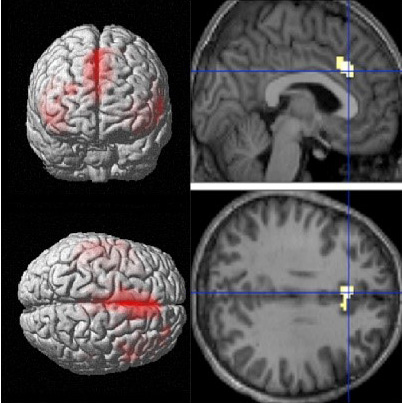 |
| fMRI scans look at how regions of the brain are connected functionally, and collect data over three dimensions. Image: Jeff Anderson. |
Another technique, functional MRI (fMRI), reveals the activation of neurons over time. fMRI recognizes an increase in oxygenation, which happens when brain cells “fire” electrical signals and quickly dissipates afterward. Anderson asks his subjects to do certain tasks (say, counting, or having a conversation), and the fMRI scan produces a movie of what happens in their brains. These movies show areas of the brain that “light up” during certain tasks.
With fMRI, Anderson creates what are called connectomes. “Brain map” doesn’t begin to describe the powerful, four-dimensional experience of a connectome.
To illustrate how a connectome works, Anderson pulls up some data from the Human Connectome Project2. He clicks on one small area in the brain – millions of neurons in a few millimeters of tissue – and the connectome shows how connected that point is to any other part of the brain he clicks on. Thanks to the precision of MRI, Anderson can move in three dimensions as he explores the brain on the screen. “Based on how bright the colors are,” he says, “you can see how connected this region is to every other region of the brain.”
The connectome really shines, though, when it is moved through the fourth dimension: time. When Anderson moves this whole picture forward one moment in time, he sees how connections change. More than simply knowing which brain areas are interacting with others, Anderson is asking how stable a connection or network is during a specific task.
“Autism is a disorder of brain connections,” explains Anderson, “so connectome research is a perfect fit.”
Anderson’s plan is to create a virtual brain, a complex digital model to simulate how autistic brains function under different conditions.
Building a Virtual Brain for Precision Medicine
Over the past 15 years, Anderson and his collaborators have built the largest longitudinal data set (same people measured over multiple points in time) of autism connectomes. They have data for 140 people with autism and 75 age-, gender-, and IQ-matched control subjects. Research reveals that people with autism show low activity in specific areas of the brain that help to recognize faces, process social function, and how you shift attention from one task to another. Anderson is proud of the work done to date. “We are world leaders in integrating fMRI with the study of autism,” he asserts.
A data set of this size opens a world of possibility. Anderson’s plan is to create a virtual brain, a complex digital model to simulate how autistic brains function under different conditions. This would be an invaluable tool in cases like autism where “you can see the brain looks normal, but the software isn’t running,” declares Anderson.
Their virtual brain will begin as a simplified connectome data set. “We’ll reduce the brain to a couple hundred or thousand points,” he explains, “and model how they’re connected. We’ll let it run and see how it behaves under different conditions.” The clear advantage to having a virtual brain for research is that they can test things without using live human subjects: how an experimental drug acts on certain brain regions, for instance.
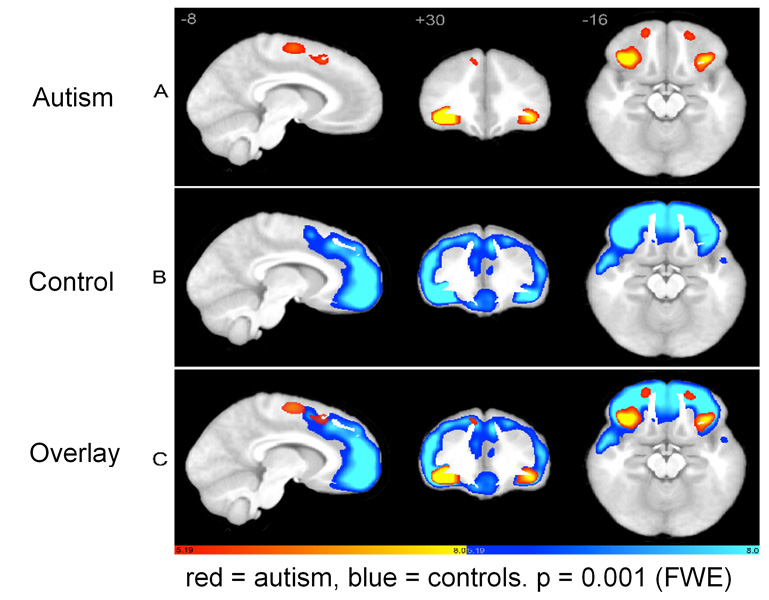 |
| People with autism show lower brain activity in specific regions as compared to control subjects. Image courtesy of Brandon A. Zielinski, MD, PhD |
The goal is personalized, precision medicine. Autism represents a wide spectrum of difficulties in social interaction, verbal and nonverbal communication, and repetitive behaviors. Some people with autism are nearly indistinguishable from “neurotypical” people and others are so severely impaired that they cannot speak or move without aid. Clearly, treatments for such a wide range of people are anything but one-size-fits-all.
“Everyone wants to know: when you scan my child, can you help us identify opportunities to remove roadblocks for their development?” he says. A custom virtual brain, loaded with data from a unique individual with autism, could be run like a simulation under different conditions and with various treatments applied to it. Watching what happens in the model would help tailor a program of suggested therapies.
“Imagine if one MRI scan could save months of heartache, worsening someone’s psychiatric condition, or even their risk of suicide,” he ponders. Anderson’s team is trying to predict who will do well and who won’t do well as they get into adulthood. “We’re trying to find the factors in the brain that make it so some people can become self-sufficient, hold down a job, and have relationships while others struggle with these things.”
A Better Definition for Mental Conditions
The “brain factors” he’s examining are not just connectome data. Diffusion tensor and structural MRI imaging allow Anderson to examine – millimeter by millimeter – which structures larger or smaller than the ones found in neurotypical brains, and understand if anatomical differences, too, are predictors of success later in life.
Anderson sums it up this way: “We’d like to measure the building blocks for psychiatric conditions.”
These quantitative “building blocks” would be invaluable to psychiatrists and pediatricians seeking a diagnosis more precise than “on the spectrum.” Today, the diagnosis of autism spectrum disorder is a rather flexible, qualitative examination of behaviors as described in the Diagnostic and Statistical Manual of Mental Disorders. If clinicians could add some quantitative measures from studies like Anderson’s, then they could provide more precise diagnoses and a clearer sense of what lies ahead in terms of treatment needs.
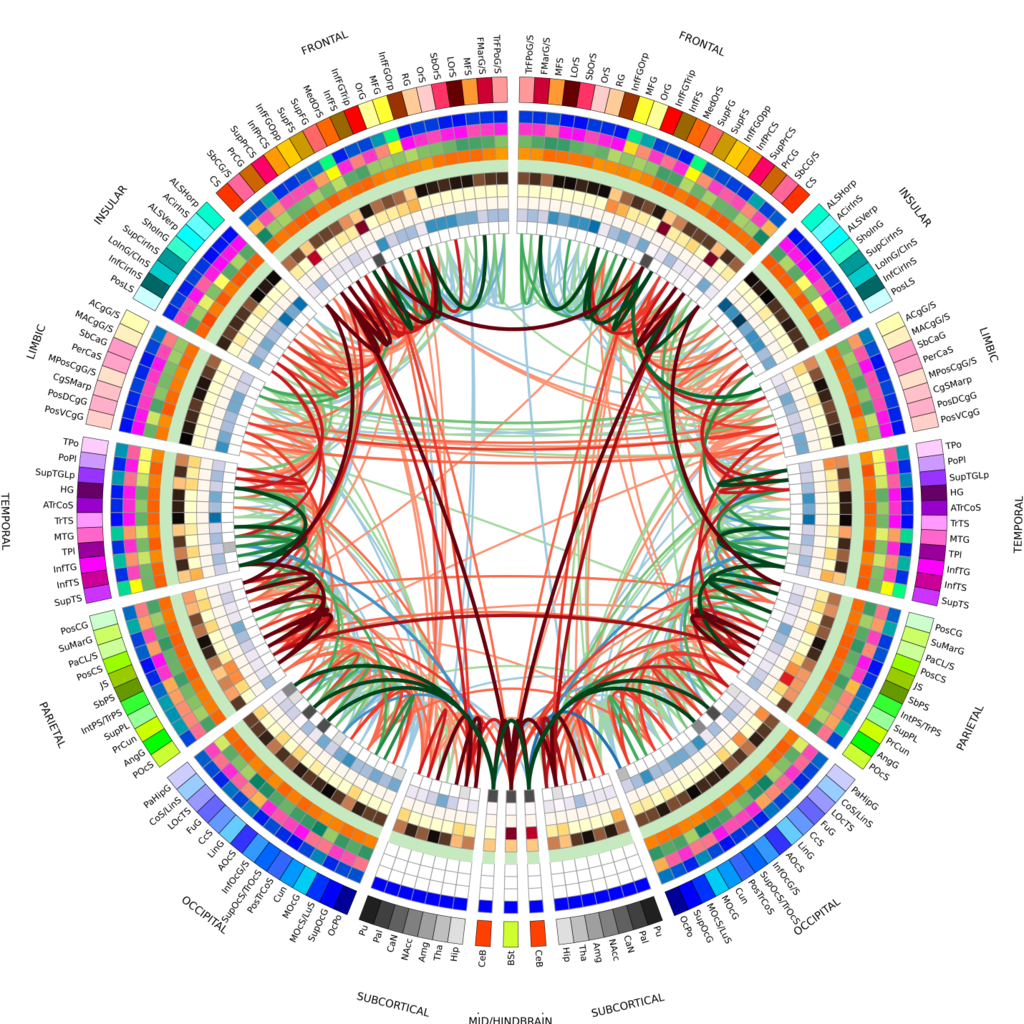 |
| A connectogram of a healthy control subject, using an additional 5 nodal measures. Image: Wikimedia Commons. |
Marrying Imaging Data and Genetic Data
Researchers have found thousands of genes implicated in the development of ASD. As genetic research continues, there is great potential for investigating how Anderson’s imaging data might relate to genetic lines. Thanks to the genealogical prowess in the state, Utah is a goldmine of genetic and health information data. The Utah Population Database contains an astounding 30,000 people with autism, and researchers at the University of Utah Health are examining the genetic data of more than 600 people with autism thanks to the Utah Genome Project.
In short, Anderson and other researchers here at the U are atop a data iceberg. The potential for collaborative discovery is enormous. With their innovative strategies and state-of-the-art technologies, there is hope for much better characterization and treatment pathways for people with autism.
1 In Utah, 1 in 58 children have ASD (one of the highest rates in the country). The US Census Bureau shows 3,000,000 people live in Utah, and 30% of them are under the age of 18. Therefore, the estimate is 3,000,000 x 0.30 x 0.01724 = roughly 15,000.
2 The Human Connectome Project was initially funded in 2009 by the National Institutes of Health to build a “network map” of the anatomic and functional connections in the healthy human brain. Nine research institutions began the project, notably University of Washington, Harvard, and UCLA, and now researchers from around the world are contributing their data.