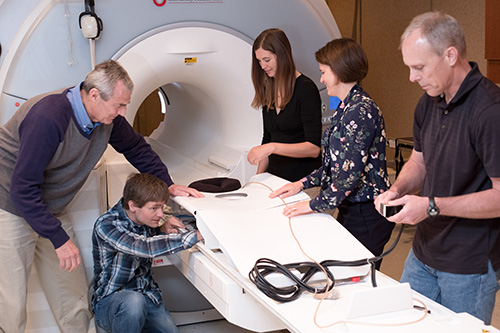
 |
| Members of the Focused Ultrasound Laboratory with the Muse focused ultrasound breast ablation device (left-to-right): Dennis Parker, PhD, Robb Merrill, Emilee Minagla, Allison Payne, PhD, Rock Hadley, PhD. |
By Michael Mozdy
A woman with a small breast tumor walks into an outpatient appointment with her radiologist, slips into a gown, and lays face-down on the table of an MRI machine. This table is built for a specific purpose – it has a space where her breast hangs suspended in water and a cradle for her forehead. The machinery under its plastic cover houses a computer-controlled, concave transducer that resembles a scuba mask. This transducer will focus the high-power ultrasound waves produced by its 256 ceramic, pencil eraser-sized elements to a precise point the size of a grain of rice.
The table and the woman slide into the bore of the MRI machine and, from a control panel outside the room, her radiologist uses MRI to locate the small tumor. After an hour of focused ultrasound waves that ablate (burn) every part of the tumor and some post-treatment imaging, the woman can put her clothes back on and walk out, tumor killed.
This might sound like Star Trek medicine, but in fact it’s a new system developed at the University of Utah Department of Radiology and Imaging Sciences by Allison Payne, PhD, and her colleagues at the Focused Ultrasound Laboratory, part of the Utah Center for Advanced Imaging Research (UCAIR). It truly is the future of non-invasive surgery –– and it’s poised for clinical trials.
From Boats to Brains to Breasts
The technology is known by many names; high-frequency focused ultrasound (HIFU) and magnetic resonance-guided focused ultrasound (MRgFUS) being the two most common. Payne also uses the simpler acronym for focused ultrasound, FUS (pronounced foos).
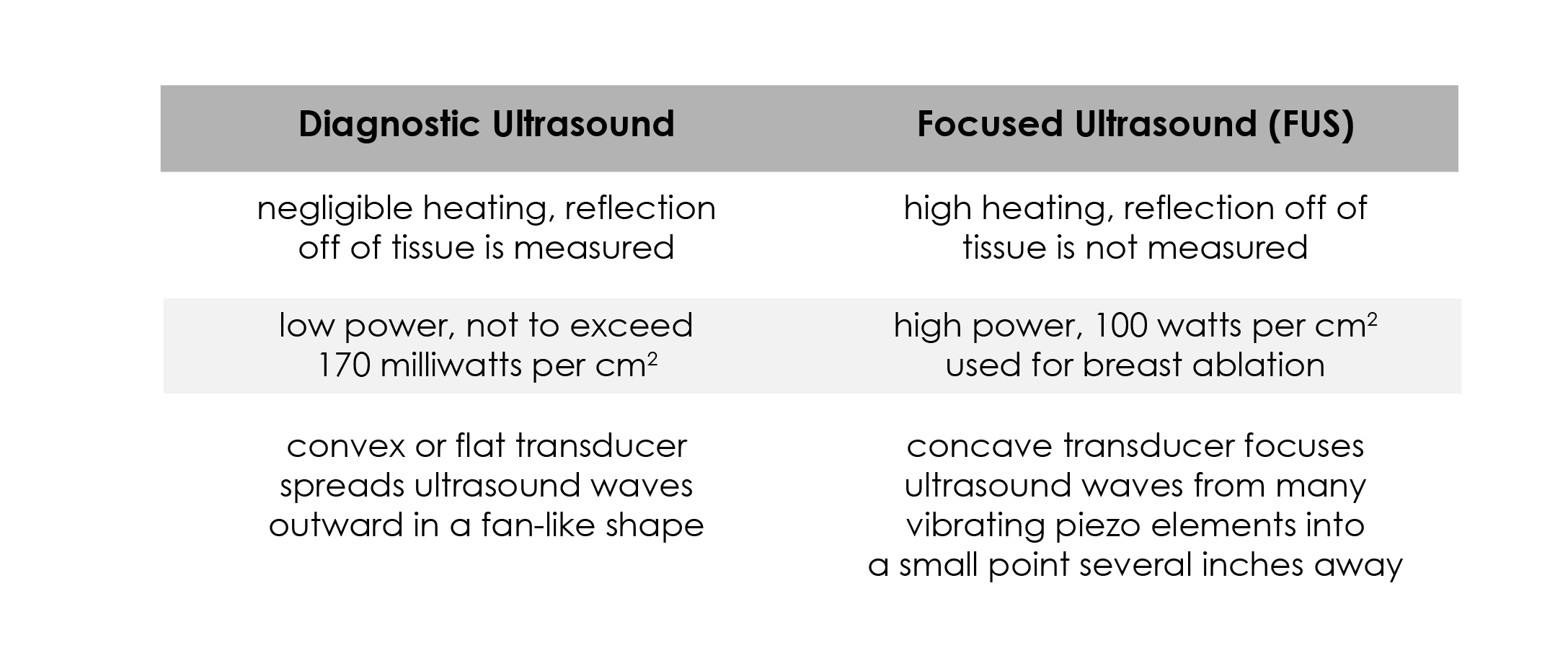
FUS should not be confused with diagnostic ultrasound, which is used throughout medicine, perhaps most famously in obstetrics where it can display a developing fetus in the womb. With diagnostic ultrasound, low-power sound waves are sent to the tissue, reflected back, and then measured and displayed. In FUS, high-powered sound waves are absorbed, turning the mechanical motion of the wave into heat (while some is also reflected back, it’s not measured with FUS applications). This type of ultrasound is interventional rather than diagnostic because – depending on the power and the amount of time used – it can slightly modify or even completely “cook” tissue.
 |
| Reginald Fessenden and the Fessenden Oscillator, an early hydrophone. In "Submarine Signaling," Scientific American Supplement, No. 2071, pp. 168-170, Sept. 11, 1915. Image from NOAA Photo Library. |
FUS has actually been around for a century – the first high frequency ultrasound device called a hydrophone was used in World War I to monitor German U boats. In the 1940s and 50s, brothers William and Francis Fry developed a way to focus this high-frequency ultrasound and burn brain tissue. But scientists couldn’t accurately see the exact location of where their focused ultrasound was being applied. So while the therapeutic potential was great, it wasn’t until the 1990s that FUS was combined with MRI technology and MRgFUS was born.
The first FDA-approved medical device using MRgFUS came in 2004, used to treat uterine fibroids. This non-invasive technology shows particular promise in treating brain disorders and disease because brain tissue is difficult to access and it is easily damaged with other treatments. In fact, MRgFUS has recently been FDA approved for essential tremor, and In fall 2017, UCAIR will have one of the first brain MRgFUS devices used for this treatment.
Payne and her mentor, Dennis Parker, PhD, realized in 2008 that focused ultrasound could offer women a completely non-invasive treatment for breast tumors with no scarring or other side effects. Nine years later, she is the first researcher in the world to develop a fully functional system customized for breast tumor ablation.
Custom Coils and a Concern for Comfort
Two aspects of Payne’s Muse system set it apart from other FUS systems: better resolution of the area to be treated thanks to custom-made equipment, and an attention to patients’ comfort.
Most FUS relies on the imaging technology “baked into” standard MRI machines. MRI works by sending and receiving radio frequency pulses while the molecules in the body are aligned along a strong magnetic field. The radio frequency pulses are sent and received via antennae coils, and these coils are located in a fixed place by the manufacturer of the MRI machine. The problem is that better images are produced when the coils are as physically close as possible to the body part being imaged. In the case of breast treatments, using the large manufacturer’s generic body coil results in adequate, but not great, images.
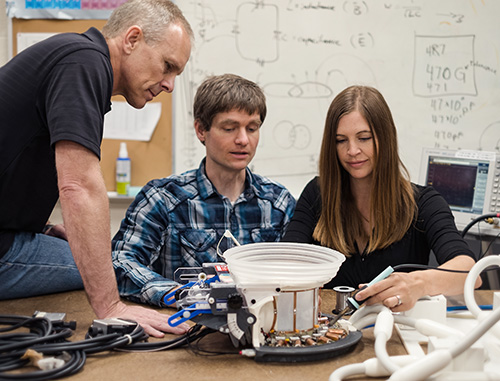 |
| Coil Lab team working on the breast-specific MRI coils for the Muse System (right-to-left): Rock Hadley, PhD, Robb Merrill, Emilee Minagla. |
Thankfully, the Radiology and Imaging Sciences Department contains a Coil Lab, run by Rock Hadley, PhD. The Coil Lab was created specifically to construct custom coils that can be used within standard MRI machines and that boost the signals being recorded – creating drastically improved images. The Coil Lab has created head coils, neck coils, and – for what Payne has dubbed the "Muse" system – breast coils that fit inside the customized table and lie much closer to a patient’s breast tissue. As a result, the Muse system can show all of the detail of the breast with great resolution and the radiologist is able to more precisely ensure the entire tumor is treated.
“Emilee [Minalga], Robb [Merrill] and Rock have worked really hard to design this coil and characterize the position of the transducer to show how that affects the signal-to-noise ratio,” relates Payne. “The coil has gone through three or four iterations to really maximize the signal.”
This custom coil also allows the radiologist to more precisely monitor temperature changes (which are measured with a special MRI algorithm) to ensure that ablation is complete, while surrounding tissues are not damaged. In the end, patients get a higher quality and safer treatment.
Complementing this technical advance, Payne has long been attuned to the challenges of asking women to lie perfectly still for upwards of 90 minutes. “Our design engineer, Robb Merrill, and engineering and design students have done an excellent job in designing the table and the head rest,” she explains, “and we’ve evaluated it with a variety of women.”
Payne doesn’t feel that their work in this area is finished. “I think it’s unique in our prototype,” she continues, “because when most people build a prototype, comfort is a secondary thought. That being said, we look forward to gathering more feedback in our clinical study and making more iterations.”
Clinical Trials as a Time for Perfection
Payne has a grant proposal out for review that will fund the beginning of human clinical trials that would eventually validate and approve the Muse system for clinical use. Part of her final research includes a verification that FUS-ablated tumor tissue has actually been killed, which requires surgical removal of some of the tissue and study of the cellular structure under a microscope. An additional part of the clinical trial will be to collect patient feedback about the Muse system and technology – determining whether sedation is helpful or not, and how the ergonomics of the system are received.
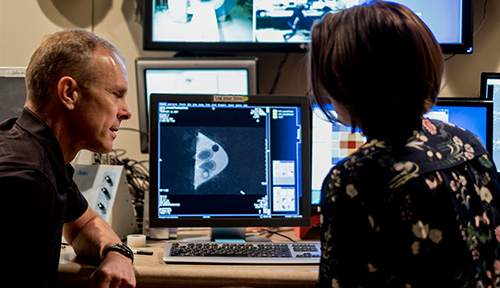 |
| Hadley and Payne at the computer control console outside the MRI room. |
At the same time, researchers are moving MRI science beyond qualitative imaging (i.e., the way tissue looks, as in a picture or movie of the tissue) to become much more quantitative. Dennis Parker, a giant in the field who continues to produce important advances from his office here in Research Park, is developing new ways to monitor MRgFUS ablation treatments in real time. In fact, he published the very first paper on using MRI to measure temperature back in 1981, and it took several years before researchers realized just how to apply this finding. More recently, he has developed a new way to simultaneously measure temperature and other MRI values that help to assess the status of tissues. These values are recorded at the level of a 1x1x3 mm box, called a voxel (the three-dimensional equivalent of a pixel).
These precise measurements are helpful when you consider what happens when you use FUS to ablate tissue: it changes in texture and sometimes shape. Also, as cells are damaged, water temporarily flows into the area. This inflammation is the fly in the ointment because it can obscure whether the ablation was completed successfully.
The Focused Ultrasound group measures their quantitative MRI values before, during, and after treatment, and thus gathers data more precise and reliable than what we can see with our eyes alone (even aided by a visual, qualitative MRI). “That gives me separate metrics of pre- and post-treatment that could correlate eventually with the outcome,” Payne explains.
Once again, the aim is to treat the tumor as precisely and thoroughly as possible, without harming any surrounding tissue. They can examine the before and after data, voxel by voxel, to ensure that the treatment was thorough and successful.
Payne comfortably switches from discussing the most technical details to focusing on the big picture ideas that propel her team forward. “We’re trying to get the best cosmetic result, the least amount of side effects, and the simplest process for women with breast tumors,” she states.
This exciting option for future breast tumor treatment is tantalizingly close, but Payne still needs funding for her clinical trials. She is hopeful that it will happen in this current round of NIH grants. When she does receive funding, we eagerly await the final steps that will make this incredible advance available to women around the world.
 |
| Focused Ultrasound Laboratory team members who have worked on the Muse system: Merrill, Minagla, Parker, Payne, and Hadley. |